The kinetic energy between two colliding particles can be expressed in terms of center of mass and relative velocity by
\[ E_k = \frac{1}{2} m_1 v_1^2 + \frac{1}{2} m_2v_2^2 = \frac{1}{2} M v_c^2 + \frac{1}{2} \mu v_r^2 , \]
a sum of a center-of-mass term and a relative momentum term.
where $M$, $v_c$, $\nu$, and $v_r$ are given by
\[ M = (m_1+m_2) ; \]
\[ \mathbf{v_c} = \frac{m_1 \mathbf{v_1} + m_2 \mathbf{v_2}}{m_1 +m_2} ;\]
\[ \mu = \frac{m_1 m_2}{m_1 + m_2} ;\]
\[ \mathbf{v_r} = \mathbf{v_1} - \mathbf{v_2} .\]
$\frac{1}{2} \mu v_r^2$ is called collision energy.
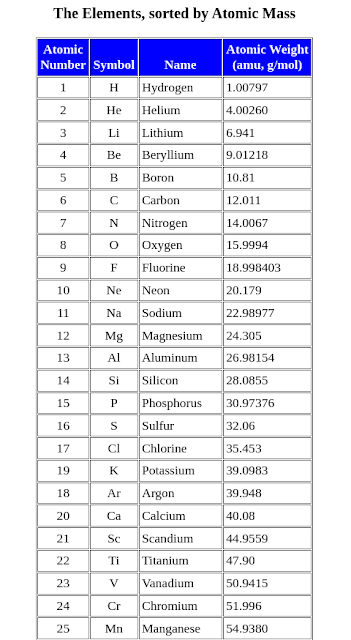
amu is short for atomic mass unit. Protons have a positive electrical charge of one (+1) and a mass of 1 atomic mass unit (amu), which is about 1.67×10−27 kilograms.
Reference:
https://web.chem.ox.ac.uk/teaching/Physics%20for%20CHemists/Mechanics/Collisions.html
https://www.angelo.edu/faculty/kboudrea/periodic/structure_mass.htm
No comments:
Post a Comment